Goal
The goal of Dr. Martínez-Cerdeño’s laboratory is to determine the etiology and the pathology of some forms of autism. In addition, we study the role of stem cells in the development, evolution, and pathogenesis of the mammalian cerebral cortex. We study the anatomy and pathology of autism and related diseases in postmortem brains and based on our findings we develop animal models for autism research. Animal models mimicking our findings in humans provide an excellent tool to generate and test new treatments for different kinds of autism.

Goal
The goal of Dr. Martínez-Cerdeño’s laboratory is to determine the etiology and the pathology of some forms of autism. In addition, we study the role of stem cells in the development, evolution, and pathogenesis of the mammalian cerebral cortex. We study the anatomy and pathology of autism and related diseases in postmortem brains and based on our findings we develop animal models for autism research. Animal models mimicking our findings in humans provide an excellent tool to generate and test new treatments for different kinds of autism.
Autism Anatomy
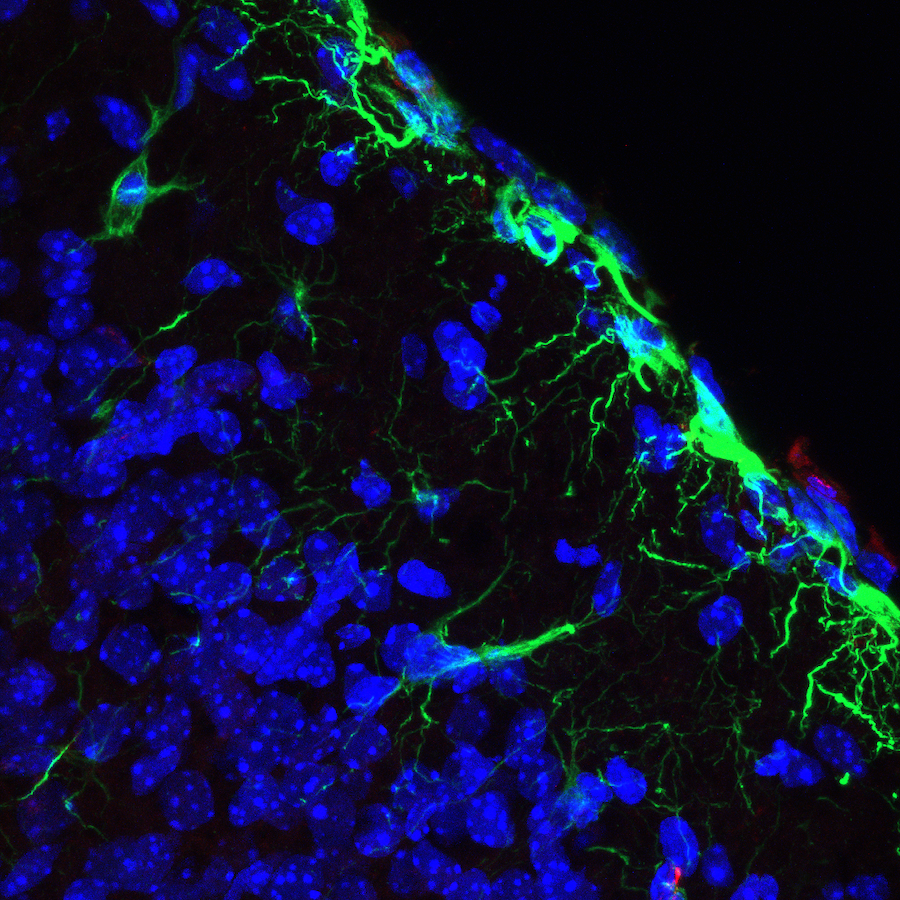
We focus on unraveling the pathology of autism. We investigate the pathology, anatomy, and histology of postmortem tissue from subjects with autism and related disorders, including Fragile X Syndrome, and Fragile X-associated tremor/ataxia syndrome (FXTAS). Our most recent discovery is that parvalbumin-expressing interneurons are reduced in discrete areas of the prefrontal cortex in autism. This discovery suggests that a deficit of inhibition acting on pyramid neurons may contribute to the cognitive phenotype of autism. This brings us a step closer to a fuller understanding of the cellular basis of autism and to the development of new therapeutic interventions.
We also developed an autoimmune mouse model of autism based on prenatal exposure to maternal antibodies. In this animal model of autism, we have shown that the anatomy of the cerebral cortex is altered by the administration of human maternal auto-antibodies during prenatal development. We found that the prenatal exposure to maternal antibodies produces behavioral changes in offspring and increased the size of neurons in the cerebral cortex. Other significant contributions in our laboratory include the discovery of an iron metabolism deficit n FXTAS, and the fact that FXTAS pathology develops in early adulthood, not late n like as previously thought.
We focus on unraveling the pathology of autism. We investigate the pathology, anatomy, and histology of postmortem tissue from subjects with autism and related disorders, including Fragile X Syndrome, and Fragile X-associated tremor/ataxia syndrome (FXTAS). Our most recent discovery is that parvalbumin-expressing interneurons are reduced in discrete areas of the prefrontal cortex in autism. This discovery suggests that a deficit of inhibition acting on pyramid neurons may contribute to the cognitive phenotype of autism. This brings us a step closer to a fuller understanding of the cellular basis of autism and to the development of new therapeutic interventions.
We also developed an autoimmune mouse model of autism based on prenatal exposure to maternal antibodies. In this animal model of autism, we have shown that the anatomy of the cerebral cortex is altered by the administration of human maternal auto-antibodies during prenatal development. We found that the prenatal exposure to maternal antibodies produces behavioral changes in offspring and increased the size of neurons in the cerebral cortex. Other significant contributions in our laboratory include the discovery of an iron metabolism deficit n FXTAS, and the fact that FXTAS pathology develops in early adulthood, not late n like as previously thought.
Autism Anatomy

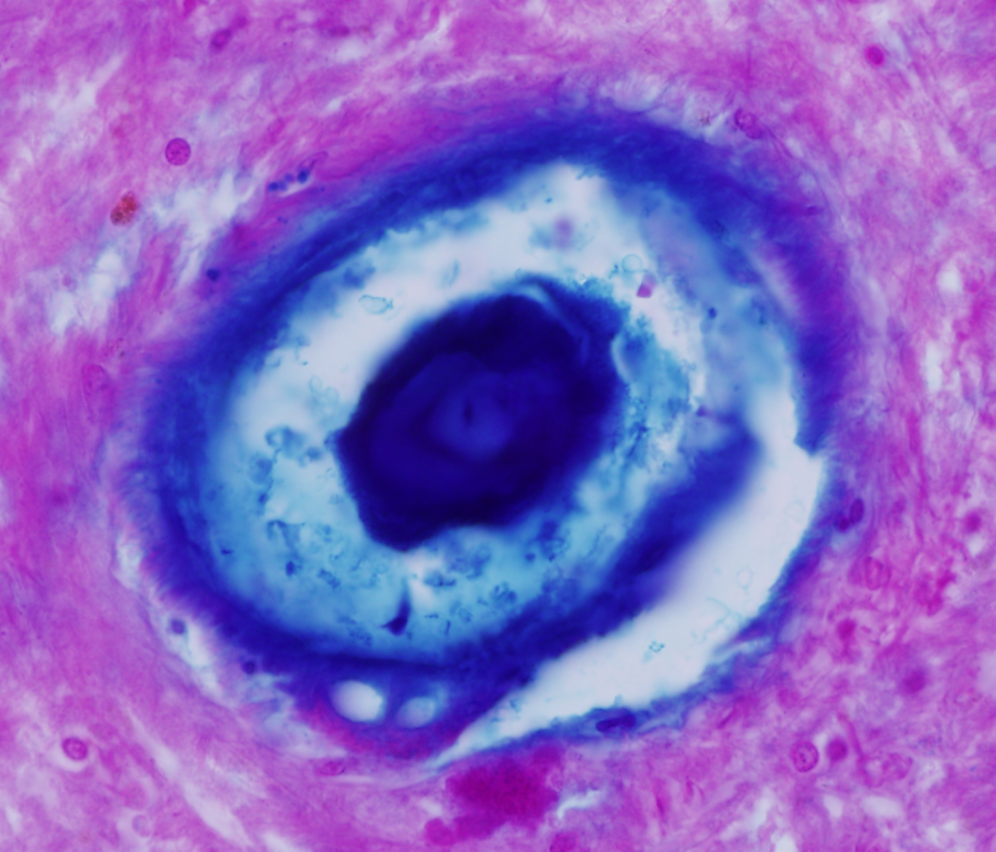
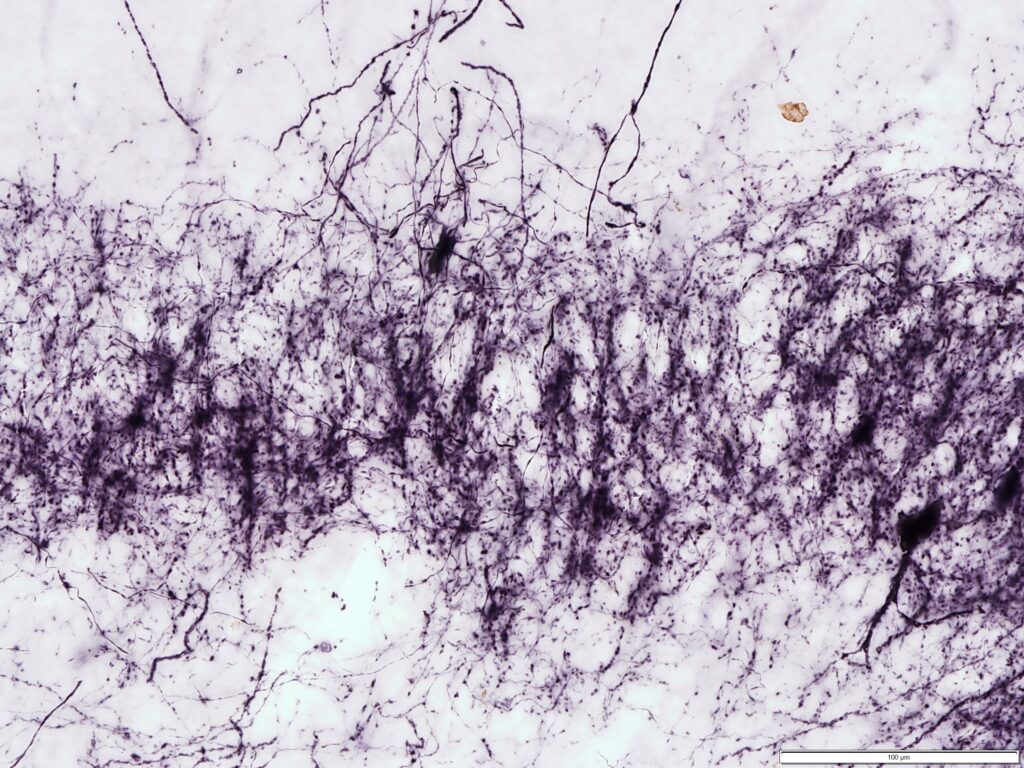
FXTAS Pathology
We are the only laboratory in the word studying the pathology and anatomy of Fragile X Tremor Ataxia Syndrome (FXTAS), a neurodegenerative disease of late onset developed by carriers of the premutation in the fragile X mental retardation 1 (FMR1) gene. We have described most of the known pathology associated to FXTAS including the presence of ubiquitin+ intranuclear inclusions Purkinje cells, iron deposition, presence of activated and senescent microglia and astrocytes, and presence of increased rate of microbleeding associated to intranuclear inclusions in endothelial cells, and Amyloid β deposition in vessels of the cerebral cortex.

FXTAS Pathology
We are the only laboratory in the word studying the pathology and anatomy of Fragile X Tremor Ataxia Syndrome (FXTAS), a neurodegenerative disease of late onset developed by carriers of the premutation in the fragile X mental retardation 1 (FMR1) gene. We have described most of the known pathology associated to FXTAS including the presence of ubiquitin+ intranuclear inclusions Purkinje cells, iron deposition, presence of activated and senescent microglia and astrocytes, and presence of increased rate of microbleeding associated to intranuclear inclusions in endothelial cells, and Amyloid β deposition in vessels of the cerebral cortex.
Cerebral Cortex Development
We contributed to a series of studies identifying a new neural precursor cell type, which we called ‘intermediate progenitor cell’ (IP), and which resides in a proliferative structure termed the sub ventricular zone. We demonstrated that estrogen regulates IP cell proliferation during neocortical development, and through comparative studies, which showed that IP cells in the prenatal brain played a crucial role in the evolution of the human cerebral cortex.
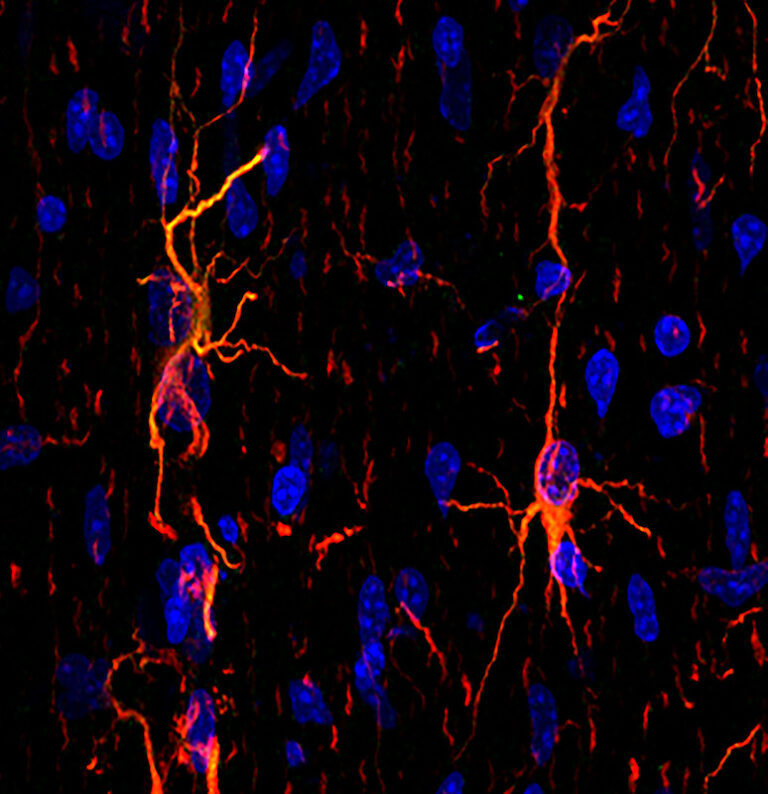
Cerebral Cortex Development
We contributed to a series of studies identifying a new neural precursor cell type, which we called ‘intermediate progenitor cell’ (IP), and which resides in a proliferative structure termed the sub ventricular zone. We demonstrated that estrogen regulates IP cell proliferation during neocortical development, and through comparative studies, which showed that IP cells in the prenatal brain played a crucial role in the evolution of the human cerebral cortex.

Cerebral Cortex Evolution
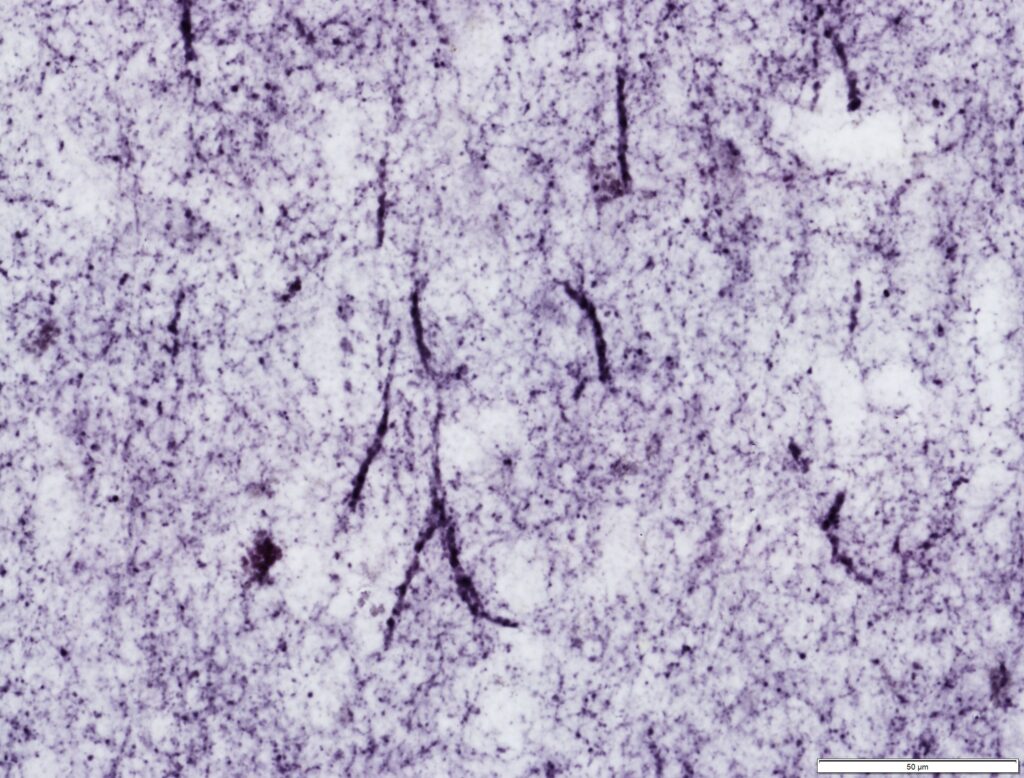
Cortical evolution has long been one of Dr. Martínez-Cerdeño’s dearest interests. Our research in cortical evolution has focused on the transition from three-layered lissencephalic cortex to six-layered gyrencephalic cortex, from both an evolutionary and a developmental perspective. One of our most notable contributions to this field, in collaboration with Dr. Stephen Noctor, was the introduction of the concept of a “two-step hypothesis of cortical evolution”. This hypothesis posits that production of excitatory cortical neurons takes place through a two-step process whereby radial glial cells produce IP cells, which then generate cortical neurons. We have provided evidence that this process evolved very early in the vertebrate brain, perhaps at the divergence of mammals and reptiles. Furthermore, we propose that this mechanism of cell generation is responsible for the expression of the cerebral cortex that took place in mammalian evolution. Since formulating this hypothesis we have published multiple papers adding novel information that corroborates the role of the two-step process of neurogenesis in the evolution of the mammalian cerebral cortex. Our interest in cortical evolution led us to establish and organize the “Cortical Evolution 2015” Conference.
Interlaminar astrocytes (ILAs) are a subset of cortical astrocytes that reside in layer I, express GFAP, contact the pia, and contain long interlaminar processes that extend through several cortical layers. We have further classified ILAs into pial and subpial ILA types, based on their soma morphology and position relative to the pia; and into typical or rudimentary, based on the length of their longer process. Our work showed that ILAs are present in the cerebral cortex of all mammalian species, have a prenatal origin from radial glial cells, they proliferate only prenatally, and that their density and morphological complexity increase during postnatal development. We demonstrated that ILAs express both stem cell (Pax6, Sox2, Nestin, Cryab, and Hopx) and astrocyte (S100β, Glast, and Aqp4) markers since early development through adult. We also showed that the morphology, developmental trajectory, and marker expression is only partially conserved between primate ILAs and mouse rudimentary ILAs.
Cortical evolution has long been one of Dr. Martínez-Cerdeño’s dearest interests. Our research in cortical evolution has focused on the transition from three-layered lissencephalic cortex to six-layered gyrencephalic cortex, from both an evolutionary and a developmental perspective. One of our most notable contributions to this field, in collaboration with Dr. Stephen Noctor, was the introduction of the concept of a “two-step hypothesis of cortical evolution”. This hypothesis posits that production of excitatory cortical neurons takes place through a two-step process whereby radial glial cells produce IP cells, which then generate cortical neurons. We have provided evidence that this process evolved very early in the vertebrate brain, perhaps at the divergence of mammals and reptiles. Furthermore, we propose that this mechanism of cell generation is responsible for the expression of the cerebral cortex that took place in mammalian evolution. Since formulating this hypothesis we have published multiple papers adding novel information that corroborates the role of the two-step process of neurogenesis in the evolution of the mammalian cerebral cortex. Our interest in cortical evolution led us to establish and organize the “Cortical Evolution 2015” Conference.
Cerebral Cortex Evolution

Interlaminar astrocytes (ILAs) are a subset of cortical astrocytes that reside in layer I, express GFAP, contact the pia, and contain long interlaminar processes that extend through several cortical layers. We have further classified ILAs into pial and subpial ILA types, based on their soma morphology and position relative to the pia; and into typical or rudimentary, based on the length of their longer process. Our work showed that ILAs are present in the cerebral cortex of all mammalian species, have a prenatal origin from radial glial cells, they proliferate only prenatally, and that their density and morphological complexity increase during postnatal development. We demonstrated that ILAs express both stem cell (Pax6, Sox2, Nestin, Cryab, and Hopx) and astrocyte (S100β, Glast, and Aqp4) markers since early development through adult. We also showed that the morphology, developmental trajectory, and marker expression is only partially conserved between primate ILAs and mouse rudimentary ILAs.
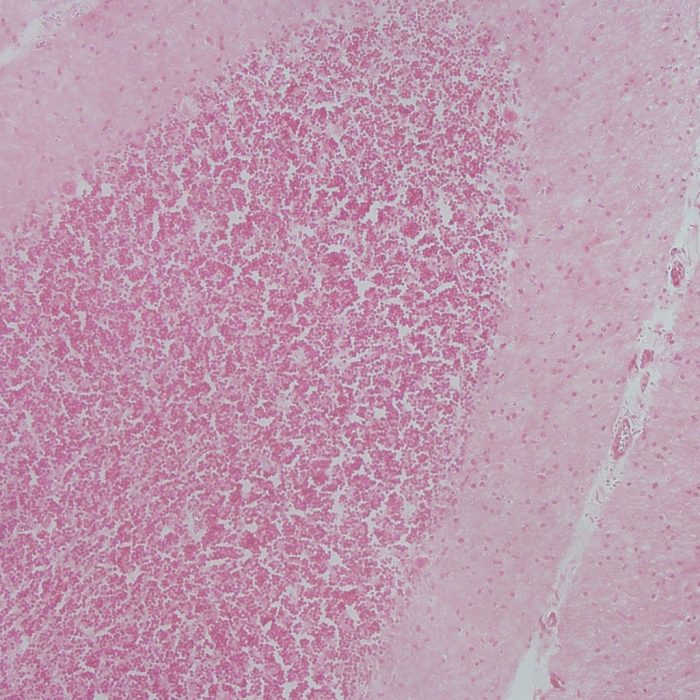
Treatments for Neurodegenerative Diseases
As a parallel line of research to our cerebral cortex studies, we have developed a line of novel treatments for neurodegenerative disease. We work on animal models of Parkinson’s disease (PD) and spinal cord injury (SCI). We developed a novel model of SCI based on the use of forceps to produce compression injuries of the spinal cord. The calibrated forceps model of compassion injury is a convenient, low cost, and very reproducible animal model that will facilitate SCI research worldwide. One of our most notable treatments is based on the use of stem cells with inhibitory neuronal fate to treat PD. This approach to treat PD, which differs from traditional approaches that focus on augmenting dopaminergic cells, has opened new avenues of research in the field of neurodegenerative treatment design.

Treatments for Neurodegenerative Diseases
As a parallel line of research to our cerebral cortex studies, we have developed a line of novel treatments for neurodegenerative disease. We work on animal models of Parkinson’s disease (PD) and spinal cord injury (SCI). We developed a novel model of SCI based on the use of forceps to produce compression injuries of the spinal cord. The calibrated forceps model of compassion injury is a convenient, low cost, and very reproducible animal model that will facilitate SCI research worldwide. One of our most notable treatments is based on the use of stem cells with inhibitory neuronal fate to treat PD. This approach to treat PD, which differs from traditional approaches that focus on augmenting dopaminergic cells, has opened new avenues of research in the field of neurodegenerative treatment design.